Clinical evaluation is one of our strengths. Shih, chi PhD is a consultant of Institutional Review Board in three Medical Center, a consultant of Taiwan Medical Care Assistive Technologies Association, a member of the Incubation Clinical evaluation, and has experience in reviewing clinical evaluation reports with health authorities (including TFDA and CDE).
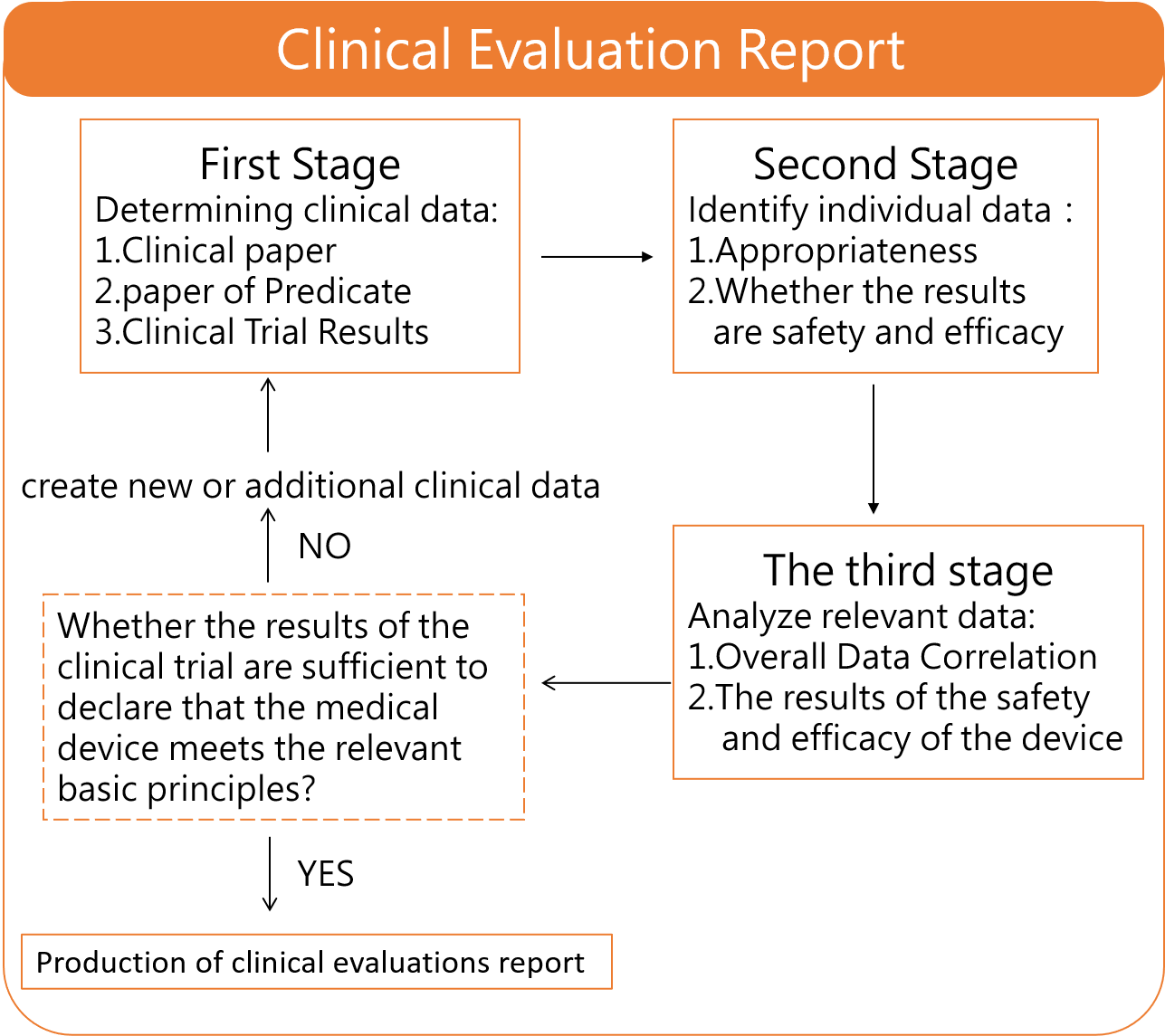
Clinical evaluation of medical device is a process to collect pre-clinical animal testing reports, clinical trial results, clinical paper of predicate, clinical experience and post-marketing clinical follow-up, which aimed to proof the acceptableness of indications, benefits and safety of medical product under normal conditions using. Based on your product , we collect Predicate data and analysis the variability, search clinical paper, integrate post-market adverse events and clinical trial data, and internal review. After preparing a clinical evaluation report that meets the requirements, we are able to answer the questions from the authorities of various countries.
License Biomedical CO.,LTD. promise to achieve all requirements efficiently.
License Biomedical CO.,LTD. promise to achieve all requirements efficiently.


